Welcome, students! In this first chapter of our electrotherapy series, we will delve into the foundational concepts of physics and the essential electrical equipment used in this field.
The knowledge shared here is derived from Clayton’s textbook on electrotherapy, which I am referencing to provide you with a simplified and clear understanding of the introduction to Electrotherapy.
This session will cover the basics you need to know as you embark on your journey in electrotherapy. We will explore critical concepts in physics that are essential for understanding how electrical equipment operates in therapeutic settings.
Topics Covered in This Session
Atoms and Atomic Structure
So, let us first understand the atom and its structure. We will try to understand what an atom is precisely.
The atom and atomic structure form the foundation of everything we see and experience in the physical world. For example, a pen and all these physical things around you, the foundation of it all, is an atom.
The atomic structures form the foundation like cells are the foundation in biology. In the same way, atoms exist in the physical world. Atoms are the foundation.
What is an atom exactly?
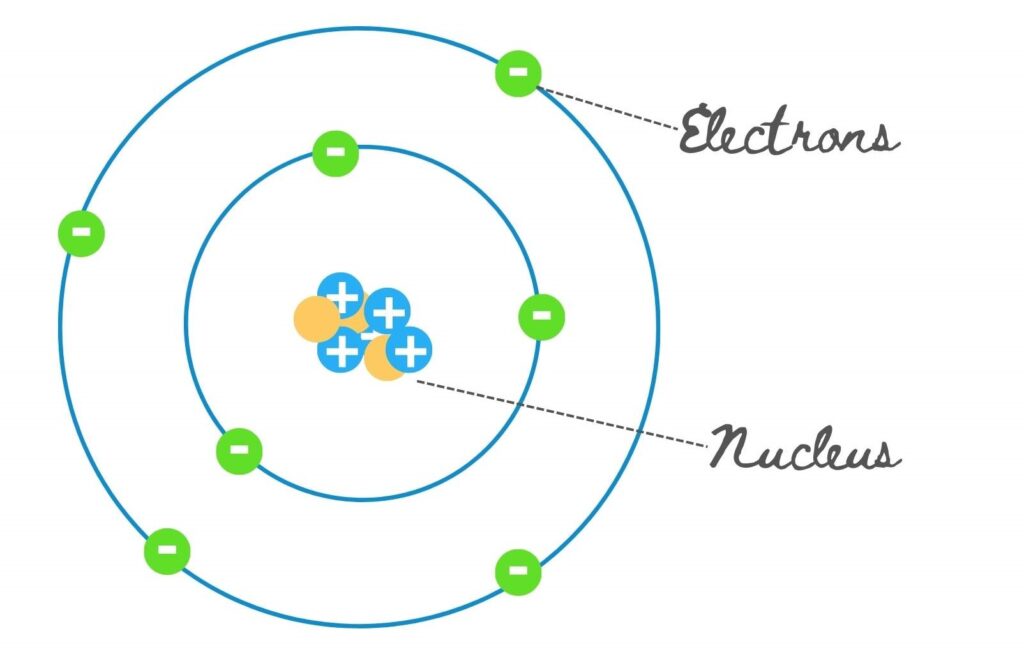
The atom comprises a central nucleus surrounded by a cloud of electrons that revolve in a definite orbit. Every electron has its definite orbit, circulating in that orbit.
We can also understand this from our solar system. The sun is at the centre of the solar system, representing the nucleus, and the planets revolve around it in fixed orbits.
Nucleus and its Components
The nucleus of an atom contains protons and neutrons. Protons are positively charged particles, which we often denote with “P” for positive.
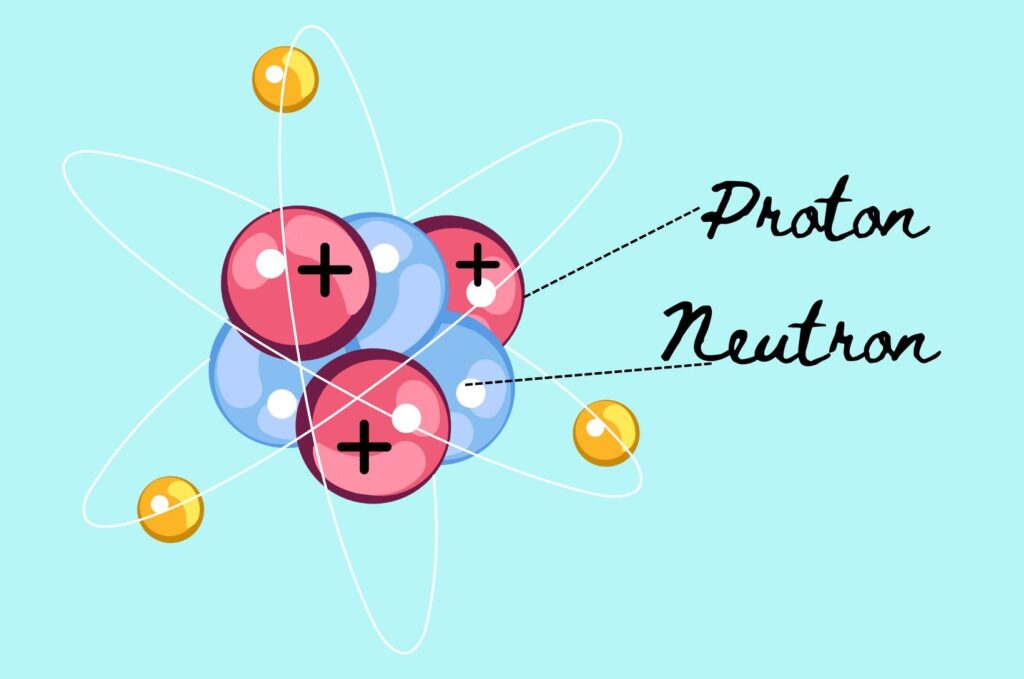
Neutrons, conversely, are neutral particles, symbolised by “N” for neutral. Strong nuclear forces hold These protons and neutrons together, keeping them compact to form the nucleus.
Negatively charged particles surround the nucleus. These electrons revolve around the nucleus in fixed orbits, creating a cloud of negative charge that influences the size and shape of the atom.
The number of electrons and the size of their orbits play a crucial role in determining the atom’s overall dimensions.
Protons are relatively large nuclear particles with a positive charge, while neutrons have a mass almost equal to protons but carry no charge. The balance between these particles within the nucleus is critical for the stability of the atom.
In the upcoming sections, we will explore how these essential components interact to create the diverse elements and compounds that form the world around us. Understanding these fundamentals is key to mastering the principles of electrotherapy and how it affects the human body.
Arrangement in the periodic table
Now, we read about the arrangement of subatomic particles. The arrangement of these subatomic particles determines the chemical properties of an element. All atomic particles—protons, neutrons, and electrons—together decide the properties of a component.
For example, the number of protons in the nucleus, also known as the atomic number, uniquely defines an element and dictates its position in the periodic table. The number of protons inside the nucleus determines its atomic number, and based on that, any element has its place in the periodic table.
If you studied chemistry, you’d know that each element has its place in the periodic table based on the number of protons inside its nucleus. The electron, on the other hand, engages in interactions with other atoms and is important for forging the bonds that form molecules and compounds.
So, what is the function of the electron?
The electron interacts between two atoms, forming a molecule or a compound. For example, if we mix kerosene and petrol, they form a compound—a combination of atoms created by the interaction of electrons.
Similarly, water (H2O) is a molecule composed of two hydrogen atoms and one oxygen atom. This interaction between atoms forms water. Understanding how particles interact with each other and how they form compounds is essential.
Introduction to Electromagnetic Radiation
Just now, we learned that electrons surround the atom’s nucleus. Electromagnetic radiation is produced when an electron rotates in a fixed orbit and leaves its orbit.
This specific type of electromagnetic wave is produced depending on each electron’s transition—where it is moving and what kind of electromagnetic wave it produces.
For example, consider tungsten.
Tungsten is a metal used in old-fashioned bulbs, where a filament is made of tungsten. When tungsten is heated, it first emits infrared waves, which can be felt as heat. Lighting up an old-fashioned bulb heats up quickly because it first emits infrared rays, making it warm to the touch.
As the tungsten gets hotter, it produces larger energy gaps, leading to the emission of electromagnetic energy in the visible spectrum. As the temperature rises, tungsten begins to emit visible light.
First, it glows red, then yellow, and eventually white as more energy is added. This process causes the metal to glow, and the light emitted falls within the visible spectrum.
So, when the tungsten filament in a bulb heats up, it starts to glow, emitting light that we can see, which is in the visible spectrum. The bulb then produces light.
Conductors and insulators
Now, we move on to the next topic: conductors and non-conductors of electricity. You must know about two types of electricity materials: conductors and non-conductors.
Conductors, like metal rods or wires, allow electricity to pass through them. For example, this pen I have is made of plastic, which does not conduct electricity, so we call it a non-conductor. But if we have a metal object, such as a rod or wire, current can pass through it, making it a conductor.
Conductors are elements that have a few loosely held electrons in their outer layer.
In conductors, some electrons in the outer layer are free, which is why they work as conductors. For instance, copper wire is a good conductor because it has loosely held electrons in its outer layer. These electrons can move freely, allowing electricity to pass through the material.
On the other hand, non-conductors, or insulators, have tightly held electrons in their outer layer, meaning they are not free to move. Examples of non-conductors include plastic, rubber, and wood. These materials cannot conduct electricity because their electrons are not free to move.
The State of Matter
The state of matter refers to the physical forms in which an element can exist: solid, liquid, or gas. This concept is fundamental in understanding how different materials interact in various environments.
For instance, water can exist in three states: solid (ice), liquid (water), or gas (steam). Each state has distinct characteristics that affect how the substance interacts with external forces, including electricity.
Latent Heat
In this section, we will discuss the concept of latent heat and the various ways heat is transmitted. These concepts are crucial in understanding how energy changes state and moves through different materials.
Latent heat refers to the internal energy required to change a substance from one state to another, such as from solid to liquid or liquid to gas. This energy is “hidden” within the molecules and is not observed as a temperature change.
For example, a specific amount of energy is required to convert ice at 0°C into water at the same temperature. This energy, known as latent heat, is necessary to overcome the bonds holding the molecules in a solid state and allow them to move more freely as a liquid.
The same principle applies when a liquid is transformed into a gas. The molecules require additional energy to break free from their liquid bonds and disperse into a gaseous state.
Understanding latent heat is essential in various therapeutic applications, especially when dealing with the effects of heat on the human body.
Transmission of Heat
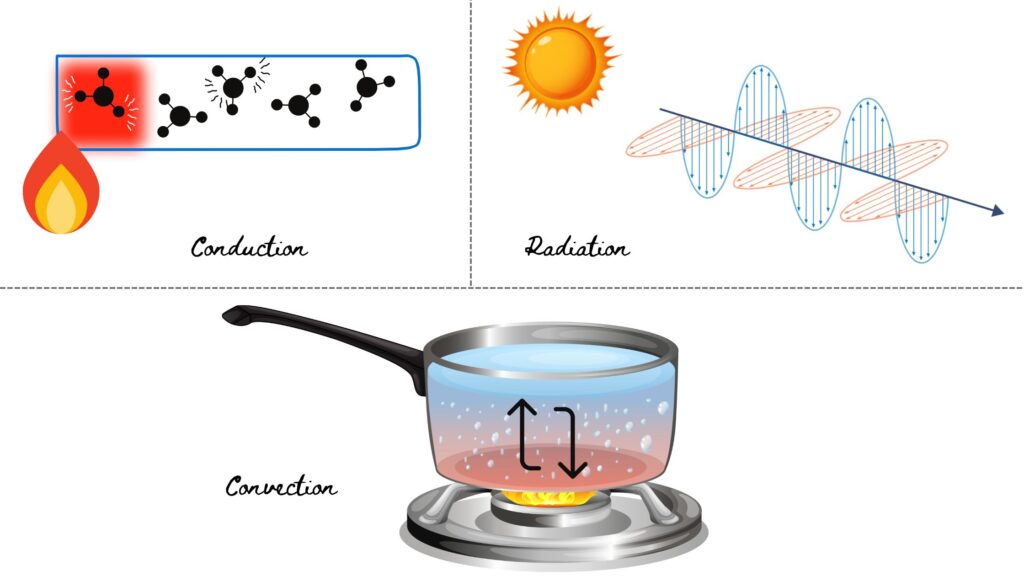
Heat transmission occurs in three primary ways: conduction, convection, and radiation.
1. Conduction:
This is the process by which heat is transferred through a solid material. For instance, when a metal rod is heated at one end, the energy causes the molecules to vibrate more intensely.
This increased vibration is then passed along to neighbouring molecules, transferring heat from the warmer end of the rod to the cooler end. Metals are particularly good conductors of heat, while materials like certain plastics are not.
2. Convection:
Convection occurs when heat is transferred through a fluid (liquid or gas) by the movement of the fluid itself. As the fluid heats up, it becomes less dense and rises, while cooler fluid takes its place, creating a circulation pattern that transfers heat.
3. Radiation:
Radiation is the transfer of heat through electromagnetic waves without needing a medium. This is how the sun’s energy reaches the Earth, and it plays a significant role in various therapeutic techniques that involve heat application.
Understanding these methods of heat transmission is vital for effectively applying electrotherapy techniques. Each method has different implications for how heat interacts with the body and how it can be used therapeutically.
Radiation, Physical Effects of Heat, and Potential Difference
In this final section, we will discuss the impact of radiation, the physical effects of heat on materials, and the concept of potential difference, all of which are integral to understanding and applying electrotherapy.
Radiation and Its Effects
Radiation occurs when specific atoms are heated, causing their electrons to move to higher energy levels. When these electrons return to their normal state, the excess energy is released through electromagnetic radiation, such as infrared radiation.
This type of radiation is crucial in various therapeutic techniques where heat is used to achieve specific physiological effects.
For example, when we apply energy to an atom, the electrons absorb this energy and jump to a higher orbit. Returning to their original orbit, they release the absorbed energy through infrared radiation.
This radiation is what conducts heat and is a fundamental concept in the application of heat in therapy.
Physical Effects of Heat
Heat has several physical effects on materials, two of which are expansion and the acceleration of chemical reactions.
1. Expansion:
When heat is applied to a material, the molecules within gain kinetic energy, leading to increased vibration. This increased movement causes the molecules to move further apart, resulting in the expansion of the material. This is why most materials expand when heated.
2. Acceleration of Chemical Reactions:
According to the laws of thermodynamics, increasing temperature accelerates the rate of chemical reactions. The added energy causes molecules to vibrate faster, leading to quicker reactions. Conversely, cooling slows the rate of chemical reactions by reducing the power available to the molecules.
Production of Potential Difference
The concept of potential difference is vital in electrotherapy. It occurs when two dissimilar metals, such as bismuth and antimony, are joined and heated at the junction.
This heating creates a potential difference between the two metals, which can be harnessed to generate an electrical current. This principle is the basis for many therapeutic devices that use electricity to stimulate healing and relieve pain.
Understanding these principles is crucial for effectively applying electrotherapy techniques. Whether you’re using heat to relax muscles or electrical currents to stimulate healing, these fundamental concepts will guide your approach and enhance the outcomes of your treatments.
Thermionic emission
In thermionic emission, the electrons are released from the molecule when a material like tungsten is heated. The material becomes rich with electrons, forming a cloud known as space charge. This process, called thermionic emission, is fundamental to the functioning of electricity.
Thermionic emission occurs, for example, when tungsten metal is heated. As energy increases, the material heats up, and with more energy, it emits light. As the energy level rises, electrons in the material start to form a cloud, creating what we call a space charge, which is an area that becomes charged. This process is known as thermionic emission.
Friends, this chapter is getting a bit long so I will divide it into two parts. In the next episode, I will teach you about static electricity, current electricity, and the electromagnetic spectrum. This will help you understand and digest the information better.
I will conclude this lecture here. I hope you enjoyed it. If you have any doubts, please comment in the comment box, and I will try to clear them. I would also appreciate your feedback on this video.
Thank you all so much for reading. Thank you!
The author is a physiotherapist who has been practising for the last 17 years. He holds a Bachelor's in Physiotherapy (BPT) from SVNIRTAR (Swami Vivekananda National Institute of Rehabilitation and Research), one of the prestigious physiotherapy schools in India.
Whatever he learns dealing with his patient, he shares it with the world through blogs and e-books. He also owns a YouTube channel, "Sunit Physiotherapist" with over 8 lakh active subscribers. Here, he shares everything he gets to learn serving the patient.